Calculate the Ph at the Equivalence Point
A sodium hydroxide NaOH b hydroxylamineNH2OH c aniline C6H5NH2. Use salt C mols saltL soln.

Ib Chemistry Higher Level Notes Acid Base Calculations
V base 0275 M.
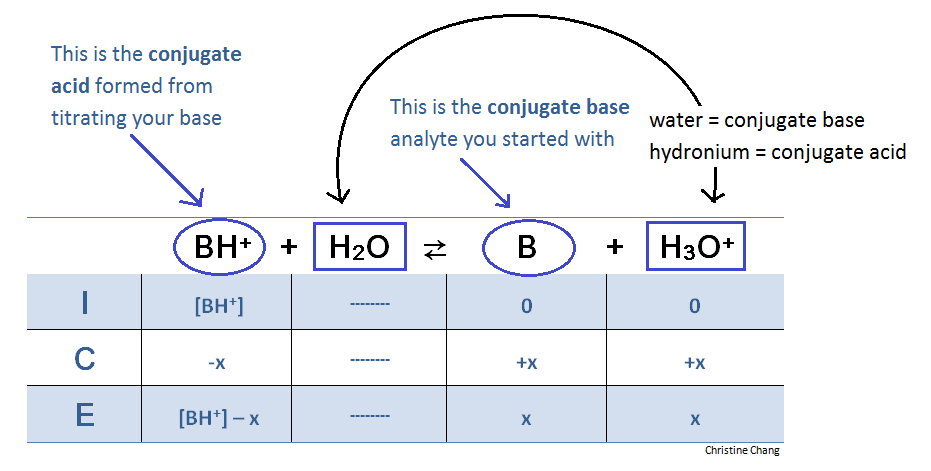
. If you titrate a weak acid eg. Initial concentration of c 0230 M at eqm c-x x x. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution.
To reach equivalence point HNO 3 KOH H 2 Ol KNO 3 aq I 0100 moles each sampletitrant C End After equivalence point any excess strong base KOH determines the pH. B C 6 H 5 NH 3 100 M pH282. Ka CH 3 COOH 18 x 10-5.
Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. CH3COOH with a strong base eg. 1 M C H 3 C O O H is titrated with a solution of 0.
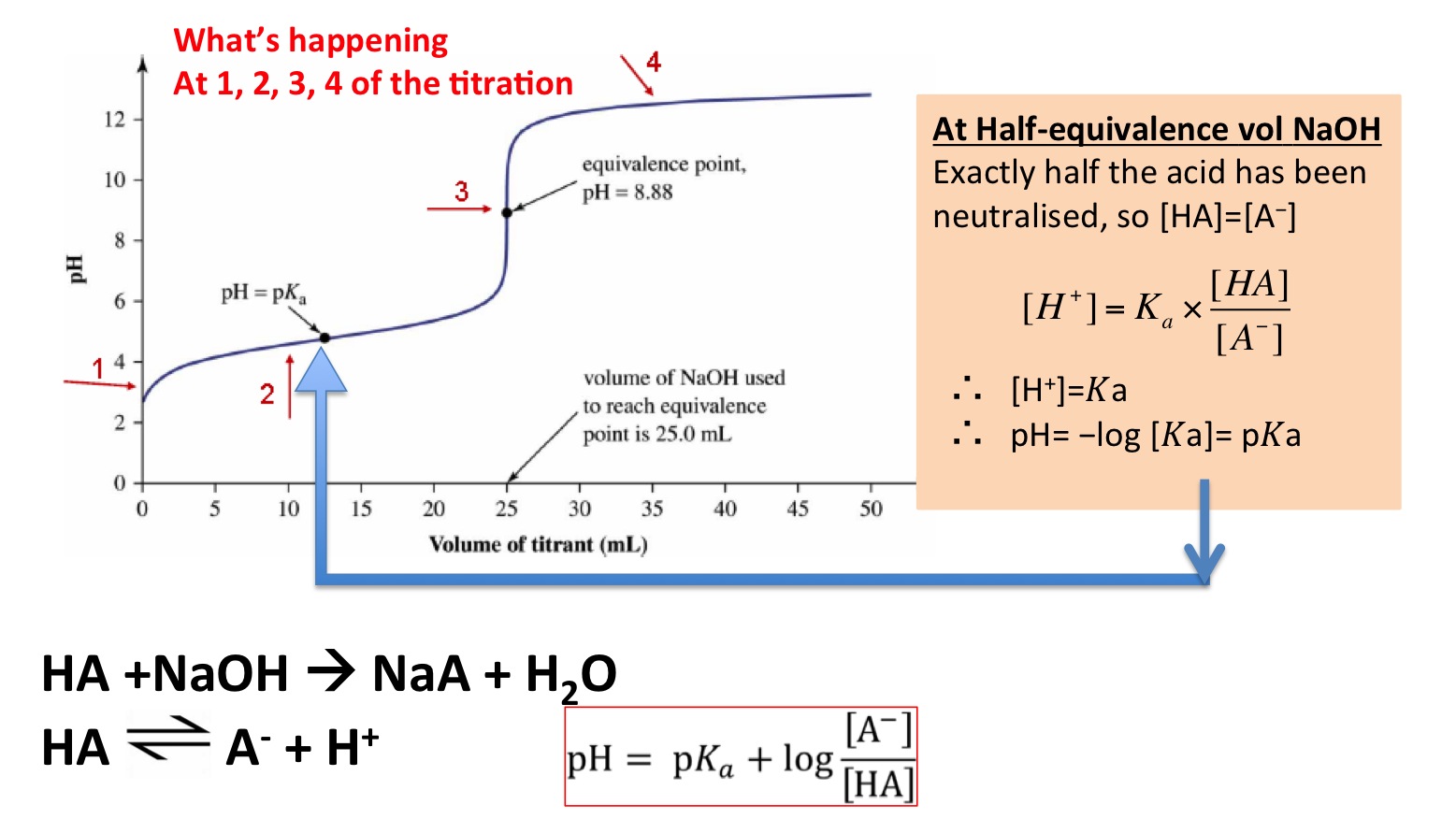
Calculate the pH at the halfway point and at the equivalence point for each of the following titrations. Calculate the pH at the equivalence point for the titration of 0110 M methylamine CH3NH2 with 0110 M HCl. M acid V acid M base V base 0275 M.
- 250-09 pH c acetic acid HC2H30 k 189-05 pH. Calculate the pH at the equivalence point in titrating 0110 M solutions of each of the following acids with a solution 0090 M in NaOH. Why is equivalence point above 7.
The Kb of methylamine is 50 104. Item 4 A Review Constants Periodic Table Part A hydrobromnic acid HB Express your answer to two decimal places. Therefore at the equivalence point even though there is no acid or base present OHH3O hence the pH will be 7.
Doceri is free in the iTunes app store. X201 x 01 K_b12 746x10-6 OH- pOH -log746x10-6 513 pH 14 - pOH 887. PH 486 Add 405 mL of NaOH pH 661 c Calculate the volume of base needed to reach the equivalence point.
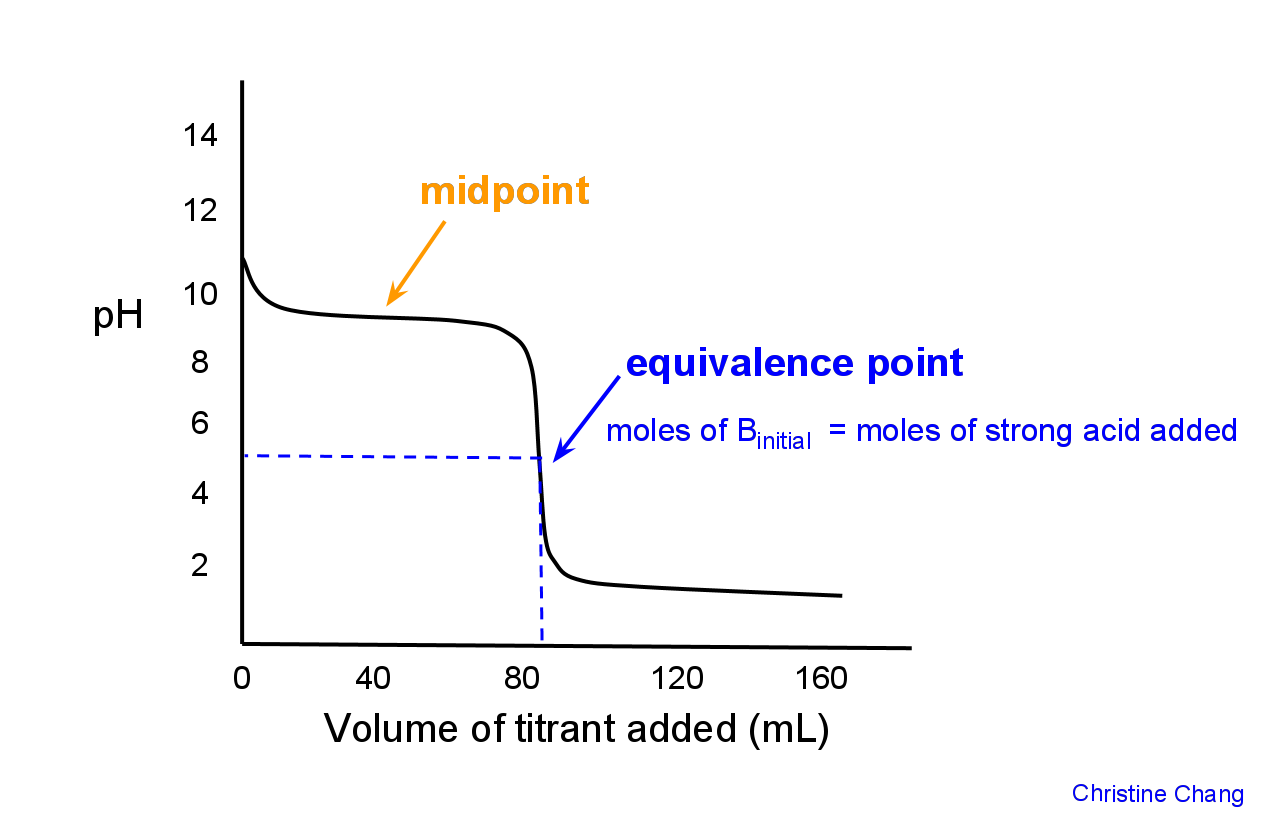
If total KOH added was 0150 moles then excess OH- 0050 moles. A nitric acid HNO3 pH 7 b hypobromous add 00 K. This is the equivalence point halfway up the steep curve.
The pH at the equivalence point for the titration will be 065. Let the concentration of be x. Calculate The Ph Of The Solution At The Equivalence Point Calculate The Ph Of The Solution At The Equivalence Point - Through the nineteen eighties Hyundai observed rapid advancement earning sizeable inroads into intercontinental markets.
For example when using a. K a of C H 3 C O O H 2 1 0 5. Calculate the pH at the equivalence point in titrating 0200 M solutions of each of the following bases with 0200 M HBr.
Kb for the A- KwKa for the acid x x C-x and solve for x OH- then convert to pH. Solving for x we get. B aniline C 6 H 5 NH 2.
At this point H O ions are completely neutralized by OH ions. 1 M K O H. The answers listed in the back of the book were.
PH -logH At equivalence point moles of acid is equal to moles of base. CH3COONa is basic and the conjugate base from the salt CH3COO- reacts with water. We can now substitute these back into the weak.
This video screencast was created with Doceri on an iPad. PH 700 Previous Answers Correct The molecular equation for the titration is as follows. In a titration it is where the moles of titrant equal the moles of solution of unknown concentration.
Since methyl-amine is a weak basecx so. A 1000 mL of 014 M HC7H5O2 Ka 64 multiplied by 10-5 titrated by 014 M NaOH halfway point equivalence point b 1000. K b is not given but we can calculate it easily from ionic product of water K w and K a via the formula K w K aK b.
Calculate the pH at the equivalence point of the titration between 01M CH 3 COOH 25 ml with 005 M NaOH. We review their content and use your feedback to keep the quality high. Nonetheless until eventually 1986 the corporation reached one among its major goals.
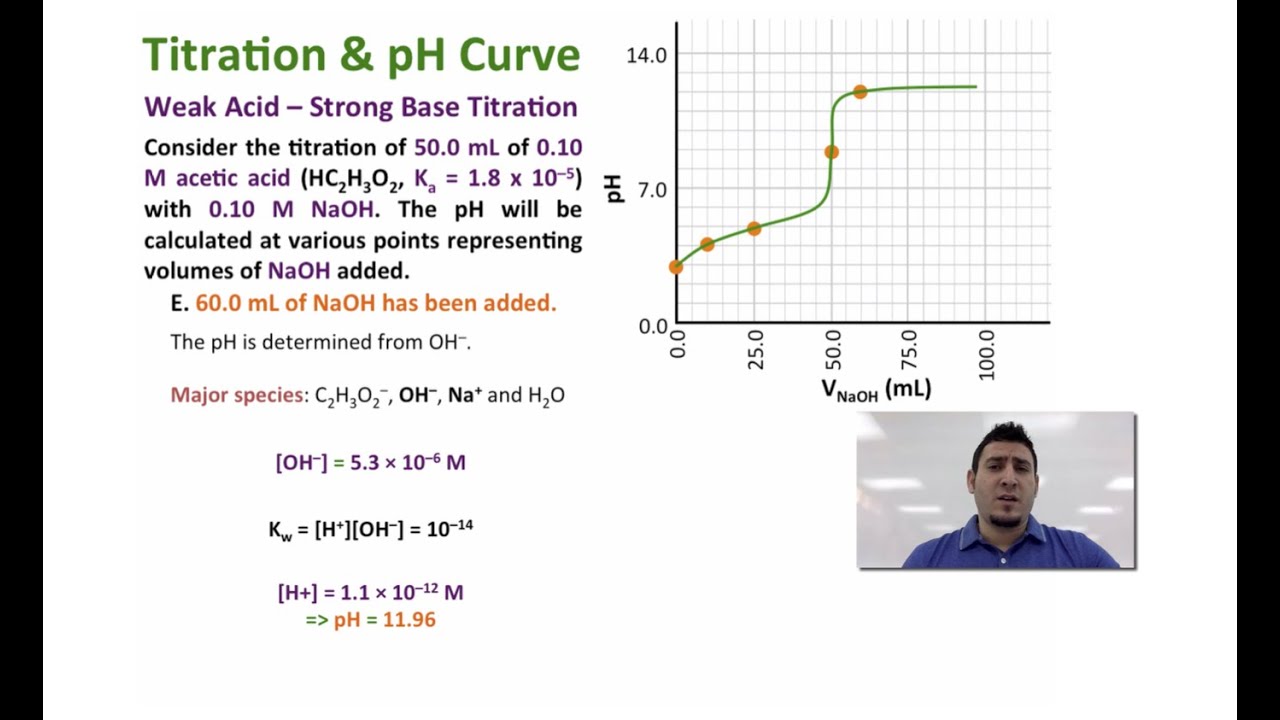
For a strong acid and a weak base the pH will be. Calculate the pH at the equivalence point of a titration between 500 mL unknown HNO2 concentration A. For the C2H5NH2 it is the salt C2H4NH3 that is hydrolyzed.
Concentration of CH 3 COO-can also be easily calculated keeping in mind the total volume is 50cm 3. Therefore we can use the formula of weak base to calculate the OH-concentration at this equivalence point. Calculate the PH at equivalence point when a solution of 0.
Using the pH of the solution a student determined that their hydroxide concentration was 01972 M. At this point moles of NaOH added moles of HCl in the analyte. K_b 556x10-10 OH-HAA- x201-x.
For this reason you must select the correct indicator for the right combination of solutions as the range of colour changes needs to have the equivalence point in it. The equivalence point will occur at a pH within the pH range of the stronger solution ie. Weak acid strong base titration.
The pH of 01 M sodium acetate is calculated as follows. This is the pH recorded at a time point just before complete neutralization takes place. 300 mL 0200 M V base 4125 mL.
300 mL 0200 M. At the equivalence point in an acid-base titration moles of base moles of acid and the solution only contains salt and water. Calculate the pH at the equivalence point in titrating 0055 M solutions of each of the following with 0044 M NaOH.
A HONH 3 100 M pH352. See pH of weak acids and bases lecture and pH cheat sheet for details of calculation. In the case of titration of weak acid with strong base pH at the equivalence point is determined by the weak acid salt hydrolysis.
A hydroxylamine NH 2 OH. The pH at the equivalence point of a monoprotic acid or monoprotic base is calculated from the hydrolysis of the salt. POH -logOH- excess pH 14 pOH Fig.
These characteristics at the equivalence point allow us to understand and simplify the formula eqpH pka logfrac left A - right left HA right eq Since eqleft HA. That means we have to find pK b of conjugated base and calculate concentration of OH-starting from there then use pH14-pOH formula. Breaking into your American market.
NaOH the salt produced eg. Calculate the pH at the equivalence point for titrating0200 M solutions of each of the following bases with 0200M HBr.
The Ph At One Half The Equivalence Point In An Acid Base Titration Was Found To Be 5 67 What Is The Value Of K A For This Unknown Acid Socratic
How Do You Calculate The Ph At The Equivalence Point For The Titration Of 190m Methylamine With 190m Hcl The Kb Of Methylamine Is 5 0x10 4 Socratic
Titration Of A Weak Base With A Strong Acid Chemistry Libretexts

Chem 123 Titration Solving For Ph At Equivalence Point Youtube
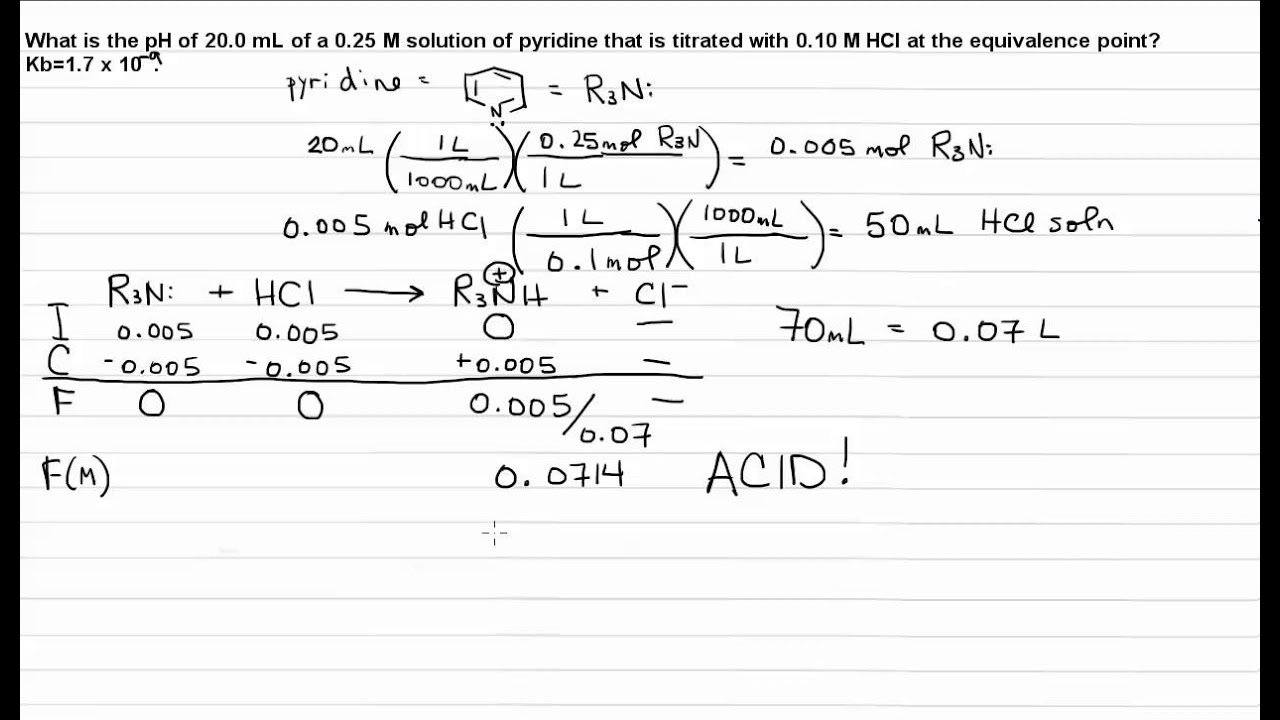
Titration Weak Base Strong Acid Equivalence Point Youtube
How Do We Determine The Right Indicator For An Acid Base Titration Do We Look At The Ph At The Equivalence Point Please Provide Examples As Well Quora

Oneclass Calculate The Ph At The Equivalence Point For The Following Titration 0 20 M Hcl Versus 0
Calculate The Ph At The Equivalence Point Of The Titration Between 0 1m Ch3cooh 25 Ml With 0 05 M Naoh Sarthaks Econnect Largest Online Education Community

Weak Acid Strong Base Titration Ph After Equivalence Point Youtube

Calculate The Ph At One Half The Equivalence Point Youtube

Acid Base Titrations Introduction 3 Overview Titrations Are Important Tools In Providing Quantitative And Qualitative Data For A Sample To Best Understand Ppt Download
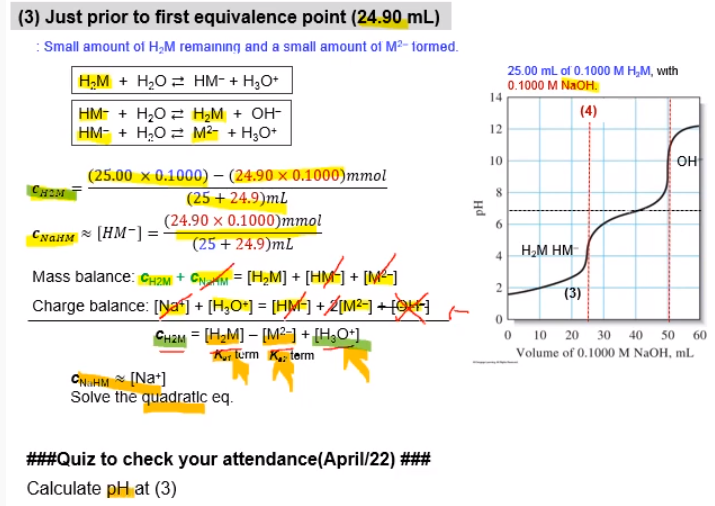
Calculate The Ph At 3 Answer Should Be Two Chegg Com

Strong Acid Strong Base Titration Ph Before Equivalence Point Youtube

Titration Of A Weak Base With A Strong Acid Chemistry Libretexts
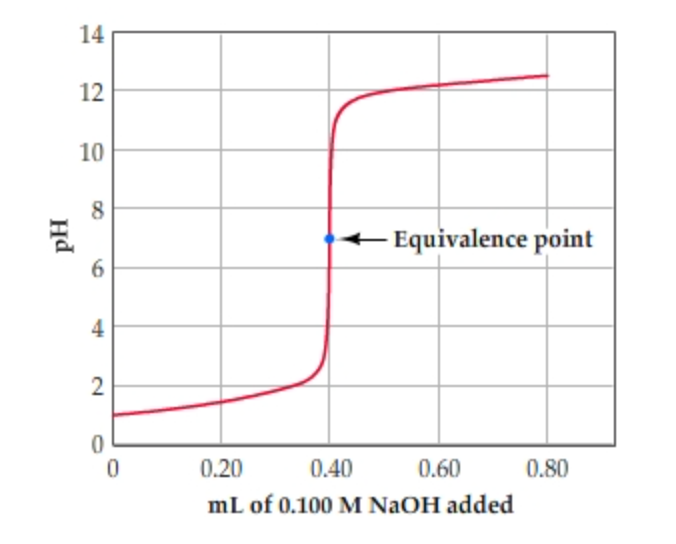
Solved Learning Goal To Calculate The Ph At The Equivalence Chegg Com

Strong Acid Strong Base Titration Ph After Equivalence Point Youtube

What Is The Approximate Ph At The Equivalence Point Of A Figure 1 Curve Lisbdnet Com

Titration Curves Equivalence Point Article Khan Academy
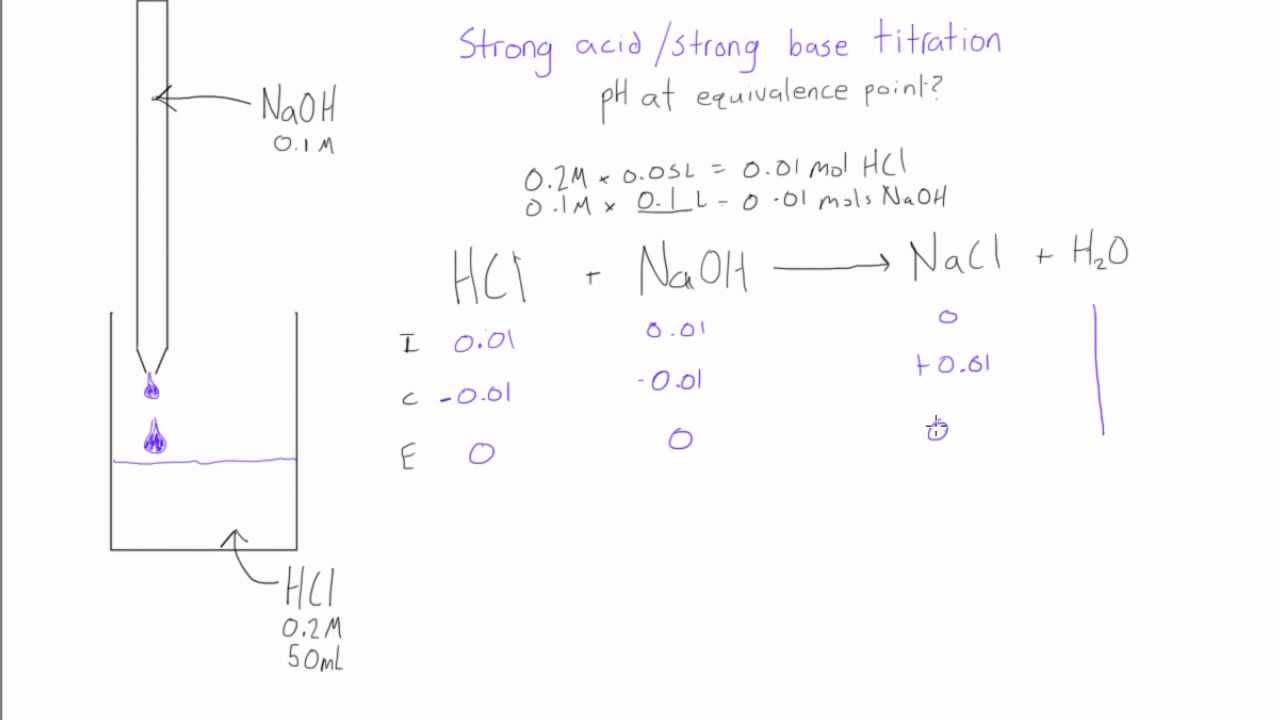
Strong Acid Strong Base Titration Ph At Equivalence Point Youtube
Comments
Post a Comment